Controlling product safety and security in times of COVID-19
25 February 2021
By Anna Abbinante, Anti-Fraud and Customs Controls Directorate, Italian Customs and Monopolies AgencyMarket surveillance system
In Italy, responsibilities for market surveillance are met by the Ministry of Economic Development (hereinafter MISE), as well as by other sector-specific agencies, such as the Ministry of Health, which is in charge of assessing the conformity of medical devices, and the Italian Medicines Agency (AIFA) and Institute for Occupational Safety (INAIL), which are in charge of assessing the conformity of Personal Protective Equipment (PPE).
Market surveillance agencies must ensure that circulating products are regularly subject to controls, either through a review of product documentation and/or (where appropriate) through physical checks and laboratory testing. However, the authority responsible for controls on products entering the European Union, in terms of their compliance with quality and safety requirements, is the Italian Customs and Monopolies Agency (hereinafter referred to as Italian Customs). The latter can suspend the release of products if it is suspected that these are unsafe and/or do not comply with the legislation, or do not fulfil documentation and marking requirements.
Where the release for free circulation is suspended, Italian Customs must immediately notify the competent market surveillance agency, which is given three working days to perform a preliminary investigation and to decide if the products can be released or must be detained for further checks on their safety and conformity. The market surveillance agency’s final decision is then notified to Customs, which releases, seizes or resends the goods accordingly.
The Standard Operational Procedures (SOP) Manual was updated in 2019 to ensure that all Customs offices apply the same procedures, and to guarantee uniform and high-quality border controls. The Manual provides Customs officers with detailed instructions on how to carry out controls, including on product safety and conformity requirements.
This operational set-up has forged strong cooperation between Italian Customs and the market surveillance agencies, and in particular with MISE. A Customs officer is seconded at MISE as a liaison officer. Given the diversity of products covered by safety and conformity requirements, assessing the compliance of a product can be a challenge. Specialized training sessions are organized for Customs officers by various specialist agencies. MISE facilitates the work of Customs by sending appropriate information on high-risk product categories, high-risk economic operators or manufacturers, and any other relevant information that will facilitate the identification of suspected unsafe or non-compliant products at the border. MISE staff also support inspection officers during physical controls, and provide advice and quick responses on technical issues. This cooperation has been formalized by an agreement between the two bodies. The other market surveillance agencies also work with Customs on the basis of similar agreements. In addition, associations of right holders provide technical support to public bodies when needed.
COVID-19
When the COVID-19 pandemic hit Italy in early 2020, Italian Customs was required to strengthen its controls on goods identified as critical in the fight against the virus, with the aim of stopping the import of illegal, unauthorized and dangerous products that could jeopardize public health. A new agreement was signed with MISE to boost Customs controls on Personal Protective Equipment and medical devices, as well as facemasks. To ensure enforcement efficiency, a series of measures were adopted.
First, risk analysis of certain types of goods was strengthened. The Customs Attaché at the Italian Embassy in China was required to provide data and information on companies authorized to produce products related to COVID-19, as well as on companies involved in fraudulent practices. To do so, the Attaché set up a direct line of communication with Chinese Customs and with the Chinese Chamber of Commerce in Italy. Italian Customs also developed risk profiles on the basis of intelligence collected nationally, as well as from other countries, the European Anti-Fraud Office (OLAF) and the WCO.
Secondly, as a temporary measure, the list of mandatory documents accompanying Customs declarations was supplemented for some products, and additional certificates sometimes required, with the aim of guaranteeing the high standard of products intended to be used in public health and by citizens. For example, a declaration related to the final destination of the goods was required. This enables Italian Customs to speed up controls on importations intended for critical private and public services, such as hospitals, retirement homes, civil protection authorities and military bodies.
Thirdly, specialized training sessions were provided for Customs officers in charge of clearing and inspecting the goods. Various services within Italian Customs were asked to provide exceptional support to field officers in order to speed up the control procedures. Teams within the market surveillance authorities (Ministry of Economic Development, Ministry of Health and Institute for Occupational Safety) seconded Customs officers to evaluate conformity with EU and national standards as per the normal procedure. The Customs Chemical Laboratory also worked hard to increase its support capacity, especially in order to assess the quality of facemasks, whose import volume increased exponentially. So too did right holders and private bodies, which even helped assess the information provided on documents and the goods themselves.
Fourthly, the number of checks on targeted goods was increased and, on a regular basis, specific instructions were provided to Customs officers to boost the efficiency and effectiveness of document-based controls and physical inspections. For instance, further instructions were provided regarding existing procedures for sending samples to the agency in charge of providing technical opinions/advice, for requesting technical support from chemical laboratories, for the handling of goods, for the safe collection of samples, and for reporting information and data for risk analysis purposes.
Fifthly, controls were also strengthened on the validity of the CE mark, and on declarations of conformity. Such controls require the consultation of “notified bodies” designated to perform specific conformity assessment procedures and to grant CE marking certificates[1]. The list of designated notified bodies is available in the Commission’s NANDO information system.
Sixthly, laboratory staff know-how, as well as testing facilities, were made available to other European Union Member States needing to assess the quality of facemasks but with little experience in this area. The existing mechanism for exchange of information between EU Member States is used to share the results of analysis (unless access to the information is restricted by the judicial authority, in which case a special authorization is needed).
On another note, considering the emergency context, Customs also had to ensure the observance of temporary export control measures adopted by the Italian Government in order to ensure that all the necessary goods and products aimed at fighting the spread of COVID-19 were available on the national market.
Results
Table 1 lists the number of products seized from January to October 2020. Most of them were sub-standard and dangerous for users. In general, forged EC certificates of conformity accompanied the import declarations.
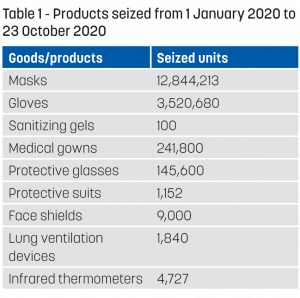
The Customs controls triggered further checks and investigations. Raids were carried out at business premises and warehouses likely to stock sub-standard, counterfeit and other illegal products. Several traders and companies were found to be unreliable operators, and their legal representatives were reported to the competent judicial authorities as alleged perpetrators of crimes against public health. Ongoing investigations are focusing on organized crime involving the trade in illegal and counterfeit products and medicines.
Special attention was given to manufacturers and distributors who used false and forged EC certificates of conformity and fake compliance certificates, and who intentionally abused business and public trust. Some companies have seen the increased demand for some products as an opportunity to make easy money, and it is important to ensure that they receive appropriate penalties


Way forward
Although no COVID-19 test kits were ever seized, Italian Customs is looking into the supply chain of such products and is collecting intelligence from other countries and from the industry to strengthen risk indicators. It is worth nothing that there are no agreed standards regarding the composition of such kits and that the authorities can therefore only seize kits if they contain dangerous or prohibited products, or on the basis of labelling and packaging infringements.
Vaccines are another product which is being carefully examined with the help of manufacturers and distributors. The main risk is the sale of fake vaccines on the internet. The most efficient way to combat such a phenomenon is to raise public awareness on the vaccine distribution policy and on the danger of these products. Italian Customs has worked in the past on communication campaigns highlighting the danger of illegal toys and medicines. Such campaigns involved public figures, such as singers and actors. A new campaign on fake vaccines will soon be launched, and Italian Customs is also working with the Italian Medicines Agency (AIFA) and health professionals to spread the message.
More information
anna.abbinante@adm.gov.it
[1] Once this assessment is done and certification received, manufacturers can add the CE mark to their products and lawfully place them on the EU market. Not all products are required to have CE marking.

